Nuclear Synthesis
Elements above iron in the periodic table cannot be formed in the normal nuclear fusion processes in stars. Up to iron, fusion yields energy and thus can proceed. But since the "iron group" is at the peak of the binding energy curve, fusion of elements above iron dramatically absorbs energy. (The nuclide 62Ni is the most tightly bound nuclide, but it is not nearly so abundant as 56Fe in the stellar cores, so astrophysical discussion generally centers on the iron.) Actually, 52Fe can capture a 4He to produce 56Ni but that is the last step in the helium capture chain.
Given a neutron flux in a massive star, heavier isotopes can be produced by neutron capture. Isotopes so produced are usually unstable, so there is a dynamic balance which determines whether any net gain in mass number occurs. The probabilities for isotope creation are usually stated in terms of a "cross-section" for such a process, and it turns out that there is a sufficient cross-section for neutron capture to create isotopes up to bismuth-209, the heaviest known stable isotope. The production of some other elements like copper, silver, gold, zirconium and lead is thought to be from this neutron capture process. It is referred to as the "s-process" by astronomers, from "slow" neutron capture.
For isotopes heavier than 209Bi, the s-process doesn't seem to work. Current opinion is that they must be formed in the cataclysmic explosions known as supernovae. In the supernova explosion, a large flux of energetic neutrons is produced and nuclei bombarded by these neutrons build up mass one unit at a time to produce the heavy nuclei. This process apparently proceeds very rapidly, in the explosion of the supernova, and is called the "r - process" for "rapid neutron capture". Chains of buildup that are not possible through the s-process happen very rapidly, perhaps in a matter of minutes, with the r-process because the intermediate products don't have time to decay.
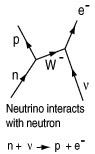 | With large neutron excesses, these nuclei would simply disintegrate into smaller nuclei again were it not for the large flux of neutrinos which make possible the conversion of neutrons to protons via the weak interaction in the nuclei. At left is the Feynman diagram for the neutrino interaction with a neutron that causes a transmutation to a proton and an electron. |
The layers containing the heavy elements may be blown off be the supernova explosion, and provide the raw material of heavy elements in the distant hydrogen clouds which condense to form new stars.
Reference
Chaisson & McMillan
Ch 21
| HyperPhysics***** Astrophysics | R Nave |