Raman Scattering
When light encounters molecules in the air, the predominant mode of scattering is elastic scattering, called Rayleigh scattering. This scattering is responsible for the blue color of the sky; it increases with the fourth power of the frequency and is more effective at short wavelengths. It is also possible for the incident photons to interact with the molecules in such a way that energy is either gained or lost so that the scattered photons are shifted in frequency. Such inelastic scattering is called Raman scattering.
Like Rayleigh scattering, the Raman scattering depends upon the polarizability of the molecules. For polarizable molecules, the incident photon energy can excite vibrational modes of the molecules, yielding scattered photons which are diminished in energy by the amount of the vibrational transition energies. A spectral analysis of the scattered light under these circumstances will reveal spectral satellite lines below the Rayleigh scattering peak at the incident frequency. Such lines are called "Stokes lines". If there is significant excitation of vibrational excited states of the scattering molecules, then it is also possible to observe scattering at frequencies above the incident frequency as the vibrational energy is added to the incident photon energy. These lines, generally weaker, are called anti-Stokes lines.
Although finding some application in vibrational spectroscopy of molecules, the use of direct infrared sources for such spectroscopy is usually much easier. Raman spectroscopy has found some application in remote monitoring for pollutants. For example, the scattering produced by a laser beam directed on the plume from an industrial smokestack can be used to monitor the effluent for levels of molecules which will produce recognizable Raman lines.
Raman scattering can also involve rotational transitions of the molecules from which the scattering occurs. Thornton and Rex picture a photon of energy slightly than the energy separation of two levels being scattered, with the excess energy released in the form of a photon of lower energy. Since this is a two-photon process, the selection rule is DJ = +/-2 for rotational Raman transitions. The sketch below is an idealized depiction of a Raman line produced by interaction of a photon with a diatomic molecule for which the rotational energy levels depend upon one moment of inertia. The upper electronic state of such a molecule can have different levels of rotational and vibrational energy. In this case the upper state is shown as being in rotational state J with scattering associated with an incoming photon at energy matching the J+2 state.
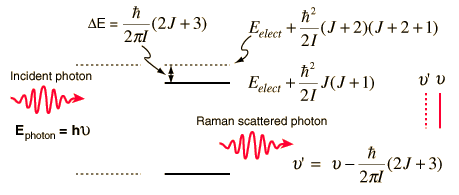
Since the Raman effect depends upon the polarizability of the molecule, it can be observed for molecules which have no net dipole moment and therefore produce no pure rotational spectrum. This process can yield information about the moment of inertia and hence the structure of the molecule.
In Raman scattering, an intense monochromatic light source (laser) can give scattered light which includes one or more "sidebands" that are offset by rotational and/or vibrational energy differences. This is potentially very useful for remote sensing, since the sideband frequencies contain information about the scattering medium which could be useful for identification. Current projects envision Raman scattering as a tool for identification of mineral forms on Mars. Such remote sensing could become a major tool in planetary exploration.
| Raman scattering from lunar soil |
| Some history |
Scattering concepts
Atmospheric optics concepts
Molecular spectra concepts
Reference
Thornton and Rex
Sec 11.1
| HyperPhysics***** Light and Vision | R Nave |