Electron Transport in the Energy Cycle of the Cell
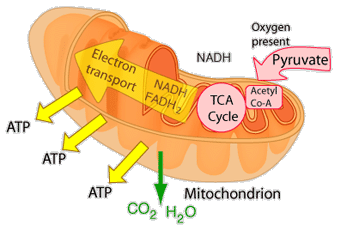 | The eukaryotic cell's most efficient path for production of vital ATP is the aerobic respiration that takes place in the mitochondria. After glycolysis, the pyruvate product is taken into the mitochondia and is further oxidized in the TCA cycle. This cycle deposits energy in the reduced coenzymes which transfer that energy through what is called the electron transport chain. |
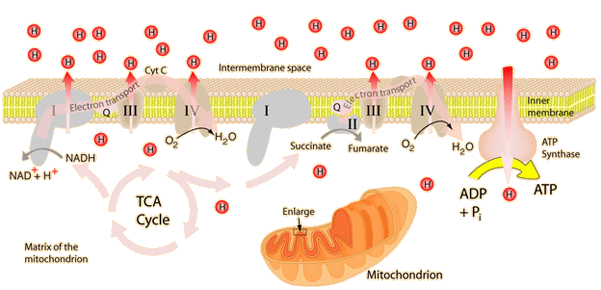 This is an active graphic.
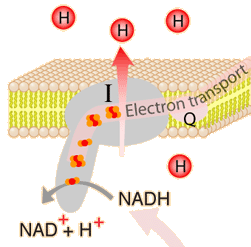
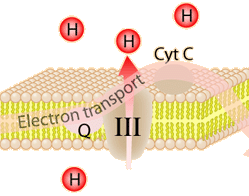
This is an active graphic.The energy given to the electrons of the reduced coenzyme NADH and to succinate by the TCA cycle is transferred in small steps in the inner membrane of the mitochondrion through a chain of five protein complexes. These small oxidation steps accomplish the conversion of ADP to the energy currency molecule ATP. This series of coupled reactions is often referred to as oxidative phosphorylation.
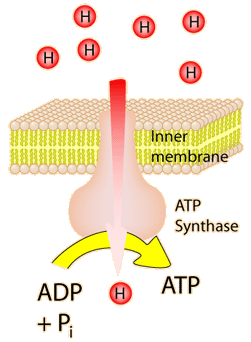
The energy used in the electron transport change pumps protons across the inner mitochondrial membrane from the inner matrix to the intermembrane space, producing a strong hydrogen concentration gradient. This process was called chemiosmosis by its discover, Peter Mitchell. This difference in proton concentration produces both an electrical potential and a pH potential across the membranes. The protein complex ATP synthase then makes use of this membrane potential to accomplish the phosphorylation of ADP to ATP.
| The Protein Complexes of the Electron Transport Chain |
| Oxidative phosphorylation wiki |
| Electron transport in the chloroplast for photosynthesis |
| Cellular Respiration |
Reference
Karp
Ch 5
| HyperPhysics***** Biology | R Nave |