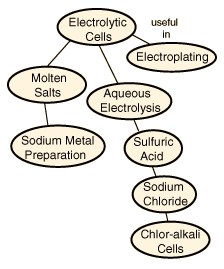
Electrolytic Cells
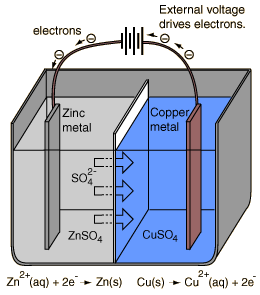
An electrolytic cell is an electrochemical cell in which the energy from an applied voltage is used to drive an otherwise nonspontaneous reaction. Such a cell could be produced by applying a reverse voltage to a voltaic cell like the Daniell cell.
 |
 |
If a voltage greater than 1.10 volts is applied as illustrated to a cell under standard conditions, then the reaction
Cu(s) + Zn2+(aq) -> Zn(s) + Cu2+(aq)
will be driven by removing Cu from the copper electrode and plating zinc on the zinc electrode.
Electrolytic processes are very important for the preparation of pure substances like aluminum and chlorine.
Electrochemistry concepts
| HyperPhysics*****Chemical concepts | R Nave |