pH
The pH of a solution is a measure of the molar concentration of hydrogen ions in the solution and as such is a measure of the acidity or basicity of the solution. The letters pH stand for "power of hydrogen" and the numerical value is defined as the negative base 10 logarithm of the molar concentration of hydrogen ions.
pH = -log10[H+]
The measurement of the pH of a sample can be done by measuring the cell potential of that sample in reference to a standard hydrogen electrode, as in the accepted procedure for measuring standard electrode potentials. This procedure would give a value of zero for a 1 Molar solution of H+ ions, so that defines the zero of the pH scale. The cell potential for any other value of H+ concentration can be obtained with the use of the Nernst equation. For a solution at 25°C this gives
or
For this expression, a base change from the natural log to the base 10 logarithm was made in the Nernst equation.
|
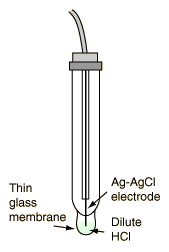
In practice, the pH is not usually measured in this way because it requires hydrogen gas at standard pressure, and the platinum electrode used in the standard hydrogen electrode is easily fouled by the presence of other substances in the solution (Ebbing). Fortunately, other practical electrode configurations can be calibrated to read the H+ ion concentration. Laboratory pH meters are often made with a glass electrode consisting of a silver wire coated with silver chloride immersed in dilute hydrochloric acid. The electrode solution is separated from the solution to be measured by a thin glass membrane. The potential which develops across that glass membrane can be shown to be proportional to the hydrogen ion concentrations on the two surfaces. In the measurement instrument, a cell is made with the other electrode commonly being a mercury-mercury chloride electrode. The cell potential is then linearly proportional to the pH and the meter can then be calibrated to read directly in pH. |
|
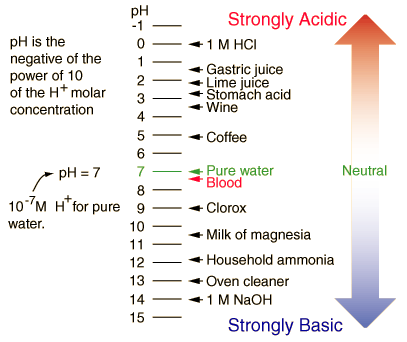
| Examples of pH values |
| Other ion concentration measurements |
Acid Concepts
Chemistry concepts
Electrochemistry concepts
Reference
Ebbing
Ch 19
| HyperPhysics | R Nave |