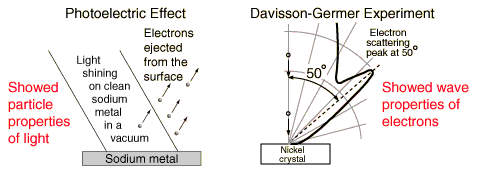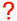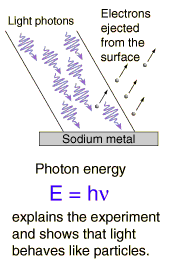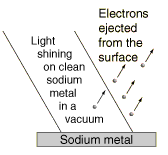
The details of the photoelectric effect were in direct contradiction to the expectations of very well developed classical physics.
The explanation marked one of the major steps toward quantum theory.
|
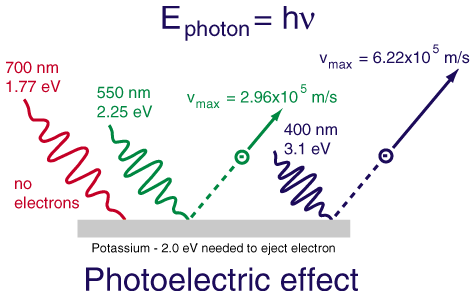
The remarkable aspects of the photoelectric effect when it was first observed were:
 | 1. The electrons were emitted immediately - no time lag! |
 | 2. Increasing the intensity of the light increased the number of photoelectrons, but not their maximum kinetic energy! |
 | 3. Red light will not cause the ejection of electrons, no matter what the intensity! |
 | 4. A weak violet light will eject only a few electrons, but their maximum kinetic energies are greater than those for intense light of longer wavelengths! |
|