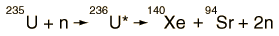Strontium-90
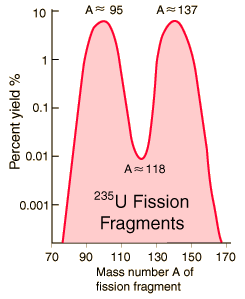
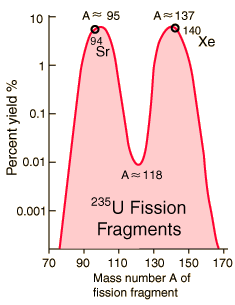
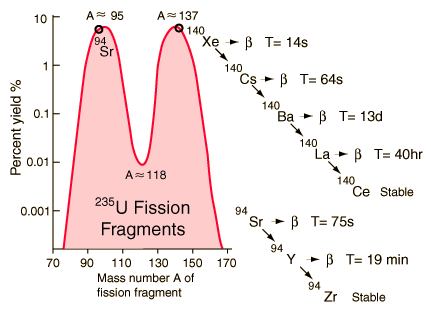
Strontium-90 and cesium-137 are the radioisotopes which should be most closely gaurded against release into the environment. They both have intermediate halflives of around 30 years, which is the worst range for half-lives of radioactive contaminants. It ensures that they are not only highly radioactive but also have a long enough halflife to be around for hundreds of years. Strontium-90 mimics the properties of calcium and is taken up by living organisms and made a part of their electrolytes as well as deposited in bones. As a part of the bones, it is not subsequently excreted like cesium-137 would be. It has the potential for causing cancer or damaging the rapidly reproducing bone marrow cells.
Strontium-90 is not quite as likely as cesium-137 to be released as a part of a nuclear reactor accident because it is much less volatile, but is probably the most dangerous component of the radioactive fallout from a nuclear weapon.
Strontium-90 undergoes beta decay, emitting electrons with energy 0.546 MeV with a half-life of 28.8 years. The decay product is yttrium-90.
References:
Environmental Protection Agency bulletin.
Wiki: Strontium-90
|