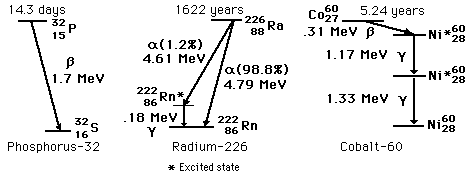
Radioactive Decay Paths
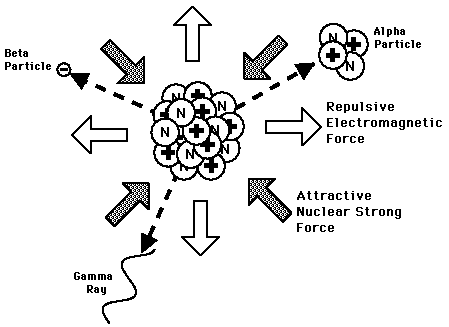
Radioactivity involves the emission of particles from the nuclei. In the case of gamma emission, the nucleus remaining will be of the same chemical element, but for alpha, beta, and other radioactive processes, the nucleus will be transmuted into the nucleus of another chemical element. Each decay path will have a characteristic half-life, but some radioisotopes have more than one competing decay path.