Starches
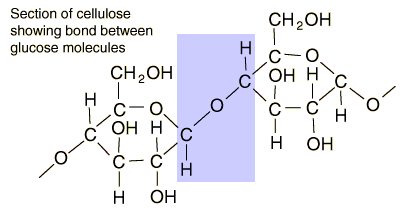
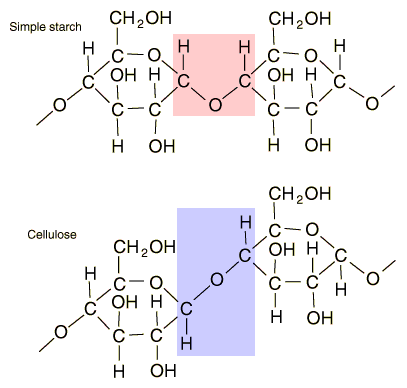
Starches are carbohydrates in which 300 to 1000 glucose units join together. It is a polysaccharide which plants use to store energy for later use. Starch forms in grains with an insoluble outer layer which remain in the cell where it is formed until the energy is needed. Then it can be broken down into soluble glucose units. Starches are smaller than cellulose units, and can be more readily used for energy. In animals, the equivalent of starches is glycogen, which can be stored in the muscles or in the liver for later use.
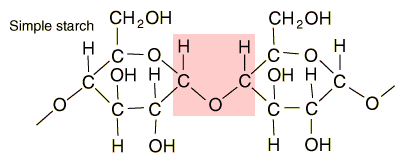
Foods such as potatoes, rice, corn and wheat contain starch granules which are important energy sources for humans. The human digestive process breaks down the starches into glucose units with the aid of enzymes, and those glucose molecules can circulate in the blood stream as an energy source. Tillery, et al. point out an interesting example of this enzyme-catalyzed breakdown process. If you chew on a piece of bread for a while, it will begin to taste sweet because the enzymes in saliva are already beginning to break down the starch into glucose, a sugar.
| Comparison of starch and cellulose |
Biochemical concepts
Chemistry concepts
Tillery,Enger, Ross
Ch 14.
| HyperPhysics*****Chemistry | R Nave |