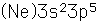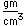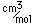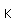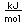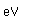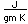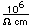Chlorine
Chlorine is the most common of the halogens. It is not as chemically active as fluorine, but is still very active and forms compounds with most elements. In pure form, it is a greenish-yellow gas with a sharp, irritating odor. It is manufactured on a large scale by electrolysis of concentrated solutions of sodium chloride.
Chlorine is a strong oxidizing agent and as a result is effective in killing bacteria. This has led to widespread use in the sterilization of drinking water, for swimming pools, etc.
Hydrogen chloride, HCl, is a gas with a sharp unpleasant odor. The gas dissolves readily in water with the liberation of heat. This forms hydrochloric acid, a very strong acid.
Chlorine is a constituent of many mineral crystals. From simple clear ionic crystals like halite, NaCl, to more complex halide containing minerals like the yellow crystals of mimetite, Pb5(AsO4)3Cl, chlorides show a range of colors.
One lead chloride mineral is mendipite, PbO2Cl2.
A chloride of copper and lead is diaboleite. A chloride of lead and antimony is nadorite, PbSbO2Cl. Lead with chlorine and fluorine form matlockite. Other lead-containing chloride minerals are boleite, cumengite and laurionite. Chlorine is in the lead-containing carbonate mineral phosgenite.
Chlorine appears in the phosphate mineral chlorapatite. It appears with copper in the sulfate mineral connellite. Chlorine is a component with mercury in the sulfate mineral kleinite. Chlorine is found in the silicate mineral marialite.
Chlorine appears in the carbonate mineral northupite.
|