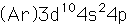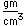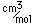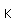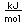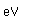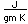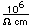Gallium
Gallium is a blue-gray metal which becomes a silvery liquid at 29.8°C. It has the rather startling property of melting in your hand. Like water, it expands on freezing. It's heat of fusion is 8.04 x 104 J/kg. Its chemistry resembles that of aluminum. It is a rare element and found little use until its properties as a semiconductor were discovered. It is used in the production of light emitting diodes (LEDs).
Gallium has been used as the liquid in high-temperature quartz-tube thermometers, which can be used to over 1200°C.
Certain gallium compounds are excellent semiconductors and have been extensively used in rectifiers, transistors, photoconductors, and laser and maser diodes.
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 28 |