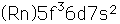Uranium Nuclear Data
| Z | A | Atomic
Mass (u) | Nuclear
Mass(GeV/c2 | Binding
Energy(MeV) | Spin | Natural
Abund. | Half-life | Decay | Q
MeV |
| 92 | 226 | 226.029170 | 210.4995 | 1725.0 | 0 | ... | 0.5s | a | 7.56 |
| 92 | 231 | 231.036270 | 215.1636 | 1758.7 | 5/2 | ... | 4.2d | EC | 0.36 |
| 92 | 232 | 232.037130 | 216.0959 | 1766.0 | 0 | ... | 68.9d | a | 5.41 |
| 92 | 233 | 233.039628 | 217.0298 | 1771.8 | 5/2 | ... | 0.159My | a | 4.91 |
| 92 | 234 | 234.040947 | 217.9625 | 1778.6 | 0 | 5.5x10-5 | 0.245My | a | 4.86 |
| 92 | 235 | 235.043924 | 218.8968 | 1783.9 | 7/2 | 0.0072 | 0.704Gy | a | 4.68 |
| 92 | 236 | 236.045563 | 219.8298 | 1790.4 | 0 | ... | 23.4My | a | 4.57 |
| 92 | 238 | 238.050785 | 221.6977 | 1801.7 | 0 | 0.99275 | 4.46Gy | a | 4.27 |
| 92 | 239 | 239.054290 | 222.6324 | 1806.5 | 5/2 | ... | 23.54m | b- | 1.26 |
Uranium plays a major role in the natural radioactive series on the earth. 235U is the progenitor of the uranium-235 series, 238U the progenitor of the uranium-238 series, and 233U is a member of the neptunium-237 series. Uranium-235 is the most important isotope for nuclear fission.
From Evans: "238U does show a half-period of about 1016 years for spontaneous fission, or about 25 fissions per hour in 1 gram of 238U. The probability of a decay is about 107 times as great." The half-life for spontaneous fission for 235U is about 1.8 x 1017 years compared to its a half-life of 7 x 108 years.
Uranium is a key to the uranium-lead dating processes in geology, where the ratio found in rock from the Earth, moon and meteorites is 238U/235U =137.88 (Dalrymple, Ch. 3). This ratio is use for developing the equations for Pb-Pb isochrons.
|