Probability for a Range of Radius
Hydrogen Ground State
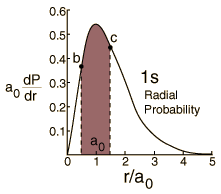 |
Finding the probability that the electron in the hydrogen ground state will be found in the range r=b to r=c requires the integration of the radial probability density. 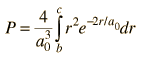 |
This requires integration by parts. The form of the solution is
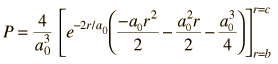
| Radial behavior of ground state | Most probable radius |
| Expectation value for radius |
Schrodinger equation concepts
Hydrogen concepts