How Quantum Numbers Arise from the Schrodinger Equation
Quantum numbers arise in the process of solving the Schrodinger equation by constraints or boundary conditions which must be applied to get the solution to fit the physical situation. The case of a particle confined in a three-dimensional box can be used to show how quantum numbers arise.
Foundational to this process is the nature of the quantum mechanical wavefunction and its root in probability. For a particle in space, the wavefunction can be written

This wavefunction is subject to the constraints

For a free particle, the x, y and z directions can be considered to be governed by independent probabilities, and the nature of probability suggests the possibility of writing the wavefunction as the product of probability densities for the three coordinates.
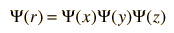
This anticipation comes from the nature of probability, and can be illustrated by the probabilities of two random events such as the throw of a die.
 | If the probability of a random event is known, then the probability of two such random events is the product of the two probabilities. In the wavefunction above, the wavefunction is written as the product of the probability densities for the x, y, and z directions. |
For a particle in a 3-D box, assume that we can write the wavefunction as
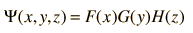
so that the Schrodinger equation becomes
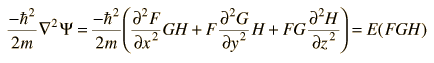
By dividing through by the wavefunction FGH, this can be put in the form
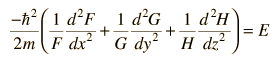
Since the equation must hold for all values of x, y and z, and since these coordinates can vary independently of each other, it follows that each term on the left must equal a constant. For example, the x part of the equation can be separated out and set equal to a constant.
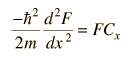
This is the form of the 1-dimensional particle in a box and has the solution
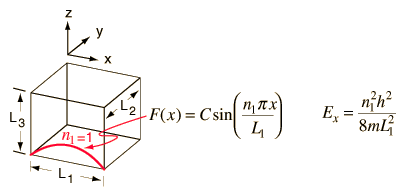
In this process, we see that the quantum number n1 arises from the nature of the wavefunction and its solution when it is forced to fit the boundary conditions imposed by the potential. Applying the same procedure to the other two dimensions leads to a solution for the wavefunction
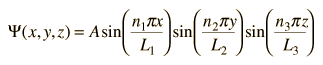
and yields energy eigenvalues which are determined by three quantum numbers.
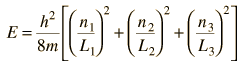
The solution to the Schrodinger equation in three dimensions led to three quantum numbers. In the case of the hydrogen atom, the boundary conditions are much different, but they also lead to three spatial quantum numbers.
Suppose the three dimensions of our box are the same. The ground state and first excited state energies are
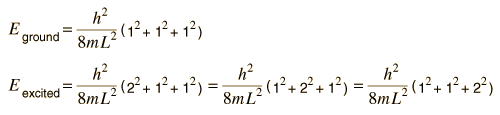
We say that the excited state is "degenerate", i.e., there are three sets of quantum numbers which give the same energy. Degeneracy in energy is generally associated with some kind of symmetry, in this case the obvious one of having cubic symmetry. In the case of the hydrogen atom, the energy level of the n=2 state depends only upon the principal quantum number. This means that it is degenerate with respect to the orbital and spin quantum numbers, an 8-fold degeneracy because of the 8 possible quantum number combinations. But this degeneracy is not exact - upon closer examination we find the hydrogen fine structure which implies a slight asymmetry introduced by the spin-orbit effect. And if we apply an external magnetic field, the Zeeman effect introduces further asymmetry and breaks up the degenerate energy levels into discrete splittings.
Schrodinger equation concepts
Hydrogen concepts
| HyperPhysics***** Quantum Physics | R Nave |