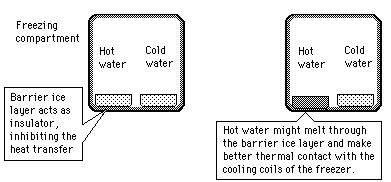
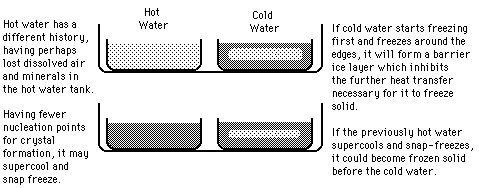
The Mpemba Effect?
There is in fact a large body of literature about initially hot water freezing faster than cold water and this phenomenon has been labeled the "Mpemba effect" (see references). The literature cited here takes the position that the phenomenon of supercooling is the main mechanism involved and that the history (dissolved gases, etc.) are not crucial to the phenomenon. Supercooling to -5 C or lower is cited and it is suggested that both the initially hot and the initially cold water supercool. If the cold water supercools more below the normal 0°C than the hot water, that will delay its freezing transition. In that case, the hot water may in fact freeze more quickly with nothing more than the degree of supercooling to distinguish the two samples. It was also pointed out that the degree of supercooling is not easily predictable, since it probably depends upon tiny particles or bubbles which nucleate the crystal formation. Experiments showed different amounts of supercooling for the same sample when it was repeatedly liquified and refrozen.
Some references are:
Knight, Charles A., The Mpemba Effect: The Freezing Times of Hot and Cold Water, Letter in Am J Phys, Vol 64, May 1996, p524
Auerbach, David, Supercooling and the Mpemba Effect: When Hot Water Freezes Faster Than Cold, Am J Phys. 63, 882-885, (1995)
Dorsey, N.E., Am. Philos. Soc. 38, 247-328, (1948).
Dorsey, N.E., The Properties of Ordinary Water Substance, Reinhold, Scranton, PA., (1940).
|
Index
Heat concepts
Heat transfer concepts |