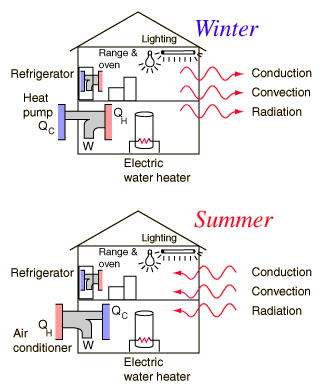
Electric Hot Water Heater
|
Commonly used electric water heaters in the U.S. use an electric resistance element to heat water directly. Some commercial water heaters employ the heat pump principle and are therefore more efficient by perhaps a factor of three, but they are not available in the residential market. Sometimes electric utility companies, in overenthusiastic marketing strategies, claim almost 100% efficiency for these water heaters. This is misleading because of the thermal bottleneck at the electric power plant; about three units of primary fuel is used to produce a unit of delivered electric energy, so the real efficiency is more like 33%. Natural gas water heaters at 60-70% efficiency are then about twice as efficient in the use of energy resources as the electric water heater.
|
The energy necessary to heat water is determined by the specific heat of water:
cwater = 1 calorie/gm °C = 4186 J/kg°C = 1 BTU/lb °F
A typical U.S. residential water heater will be taken as one which has a capacity of 40 U.S. gallons (= 320 pounds, 145 kg, 151 liters). The typical heating range will be taken to be from 60 °F to 140 °F (15.6 °C to 60 °C). The energy required to heat the water can be determined from the specific heat relationship.
Q = cmΔT
The energy required to heat one tank of water over the specified range is then
(1 BTU/lb °F)(320 lb)(140°F - 60 °F) = 25,600 BTU
or
(4186 J/kg°C)(145 kg)(60 °C - 15.6 °C) = 26.9 million Joules
Since a kilowatt-hour is 3.6 million Joules, this energy amounts to about 7.5 kWh of electricity. Taking an electric energy cost of 9.5¢/kWh, it would cost about 71¢ to heat one tank of hot water with an electric hot water heater assuming all the electric energy went into heating the water.
|