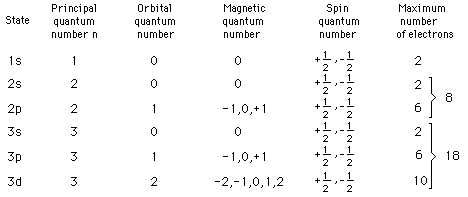Atomic Properties
The electrons associated with atoms are found to have measurable properties which exhibit quantization. The electrons are normally found in quantized energy states of the lowest possible energy for the atom, called ground states. The electrons can also exist in higher "excited states", as evidenced by the line spectra (e.g. the hydrogen spectrum) observed when they make transitions back to the ground states. The existence of these excited states can be demonstrated more directly in collision experiments like the Franck-Hertz experiment .
Other properties associated with the electron energy levels such as orbital angular momentum and electron spin are also quantized and give rise to the quantum numbers used to characterize the levels. These quantized properties are associated with periodic table of the elements, and the requirements of the Pauli exclusion principle on the quantum numbers can be viewed as the origin of the periodicity. The periodic table provides a convenient framework for cataloging other physical and chemical properties of atoms.
While the hydrogen electron energy levels are found to depend only upon the principal quantum number, the energy levels in other atoms are found to have strong dependence upon the orbital quantum number. These levels show a smaller amount of dependence upon the total angular momentum. This dependence may arise from interactions within the atom such as the spin-orbit interaction or may arise only when external fields are applied. When magnetic fields are applied, there is splitting of atomic energy levels from the Zeeman effect, and in response to electric fields there is splitting called the Stark effect.
|