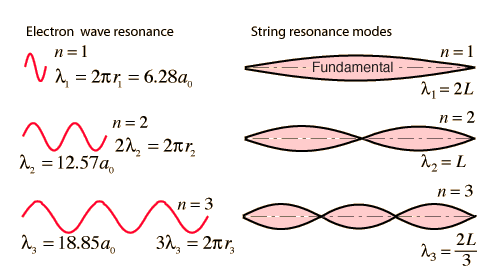
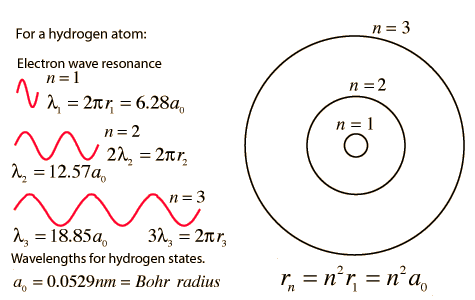
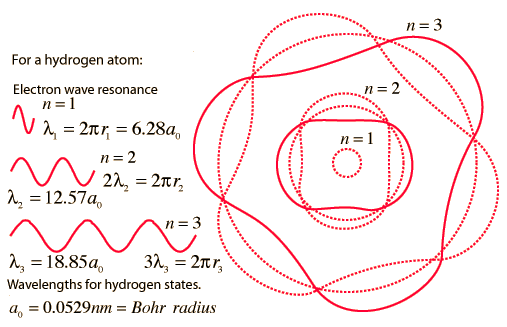
The Line Spectrum Problem
In the years before the beginning of the 20th century, the light emitted from luminous gases was found to consist not of a continuous range of wavelengths, but of discrete colors which were different for different gases. These spectral "lines" formed regular series and came to be interpreted as transitions between atomic energy levels. This presented a considerable problem for classical physics, because accelerated charges were known to radiate electromagnetic energy. It was expected that orbits of electrons about positive nuclei would be unstable because they would radiate energy and therefore spiral into the nucleus. No classical model could be found which would yield stable electron orbits.
The Bohr model of the atom started the progress toward a modern theory of the atom with its postulate that angular momentum is quantized, giving only specific allowed energies. Then the development of the quantum theory and the Schrodinger equation refined the picture of the energy levels of atomic electrons.
|