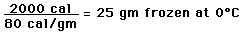Cooling a Cup of Coffee
You have a 200 gram cup of coffee at 100 C, too hot to drink. Various cooling strategies demonstrate specific heat, phase changes, and the approach to thermal equilibrium.
|
 |
|
Heat transfer concepts
Heat transfer examples
| HyperPhysics***** Thermodynamics | R Nave |
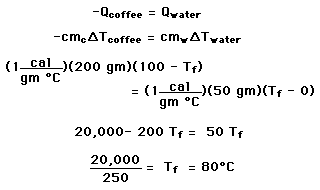

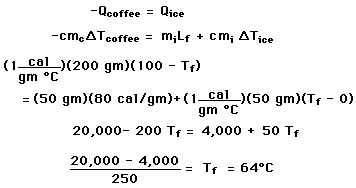


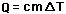 is valid only so long as a
is valid only so long as a