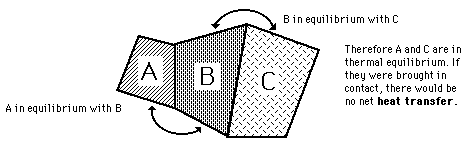
Thermal Equilibrium
It is observed that a higher temperature object which is in contact with a lower temperature object will transfer heat to the lower temperature object. The objects will approach the same temperature, and in the absence of loss to other objects, they will then maintain a constant temperature. They are then said to be in thermal equilibrium. Thermal equilibrium is the subject of the Zeroth Law of Thermodynamics.
Heat transfer concepts
| HyperPhysics***** Thermodynamics | R Nave |