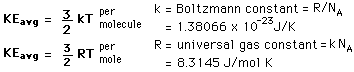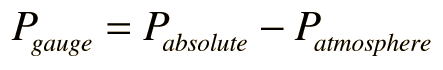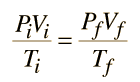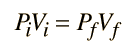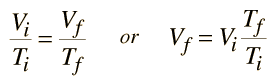Ideal Gas Law
An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in which there are no intermolecular attractive forces. One can visualize it as a collection of perfectly hard spheres which collide but which otherwise do not interact with each other. In such a gas, all the internal energy is in the form of kinetic energy and any change in internal energy is accompanied by a change in temperature.
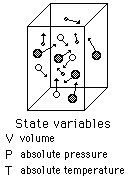
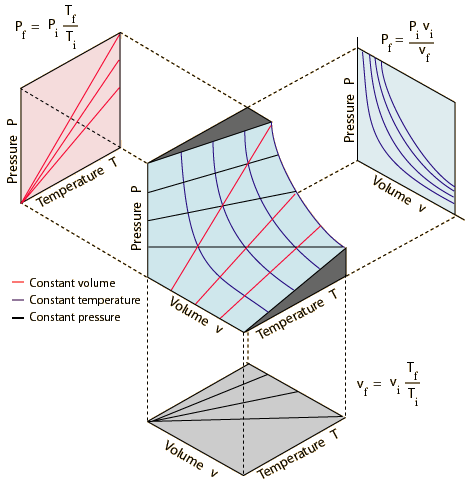
An ideal gas can be characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them may be deduced from kinetic theory and is called the
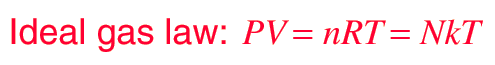
- n = number of moles
- R = universal gas constant = 8.3145 J/mol K
- N = number of molecules
- k = Boltzmann constant = 1.38066 x 10-23 J/K = 8.617385 x 10-5 eV/K
- k = R/NA
- NA = Avogadro's number = 6.0221 x 1023 /mol
The ideal gas law can be viewed as arising from the kinetic pressure of gas molecules colliding with the walls of a container in accordance with Newton's laws. But there is also a statistical element in the determination of the average kinetic energy of those molecules. The temperature is taken to be proportional to this average kinetic energy; this invokes the idea of kinetic temperature. One mole of an ideal gas at STP occupies 22.4 liters.
| Calculation |
| Departure from an ideal gas: van der Waals equation of state |
Gas law concepts
Kinetic theory concepts
| HyperPhysics***** Thermodynamics | R Nave |