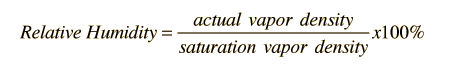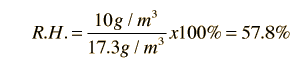How much moisture can the air "hold"?
| Careful! There are dangers and possible misconceptions in these common statements about relative humidity. |
| Relative humidity is the amount of moisture in the air compared to what the air can "hold" at that temperature. When the air can't "hold" all the moisture, then it condenses as dew. |
|
Of all the statements about relative humidity that I have heard in everyday conversation, the above is probably the most common. It may represent understanding of the phenomenon, and has some common sense utility, but it may represent a complete misunderstanding of what is going on physically. The air doesn't "hold" water vapor in the sense of having some attractive force or capturing influence. Water molecules are actually lighter and higher speed than the nitrogen and oxygen molecules that make up the bulk of the air, and they certainly don't stick to them and are not in any sense held by them. If you examine the thermal energy of molecules in the air at a room temperature of 20°C, you find that the average speed of a water molecule in the air is over 600 m/s or over 1400 miles/hr! You are not going to "hold" that molecule!
Another possibly helpful perspective would be to consider the space between air molecules under normal atmospheric conditions. From knowledge of atomic masses and gas densities and the modeling of the mean free path of gas molecules, we can conclude that the separation between air molecules at atmospheric pressure and 20°C is about 10 times their diameter. They will typically travel on the order of 30 times that separation between collisions. So water molecules in the air have a lot of room to move about and are not "held" by the air molecules.
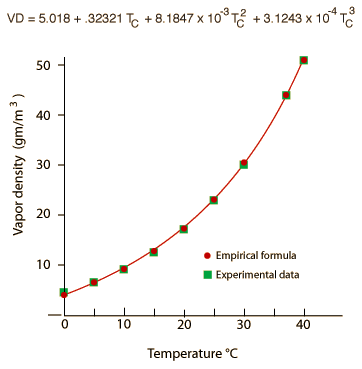
When one says that the air can "hold" a certain amount of water vapor, the fact that is being addressed is that a certain amount of water vapor can be resident in the air as a constituent of the air. The high speed water molecules act, to a good approximation, as particles of an ideal gas. At an atmospheric pressure of 760 mm Hg, you can express the amount of water in the air in terms of a partial pressure in mm Hg which represents the vapor pressure contributed by the water molecules. For example at 20°C, the saturation vapor pressure for water vapor is 17.54 mm Hg, so if the air is saturated with water vapor, the dominant atmospheric constituents nitrogen and oxygen are contributing most of the other 742 mm Hg of the atmospheric pressure.
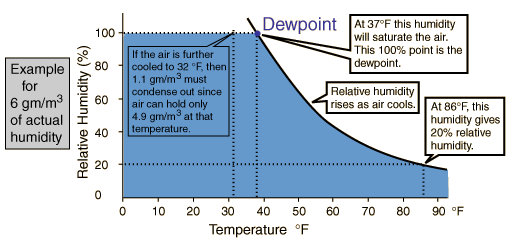
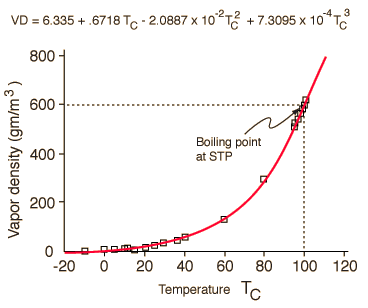
But water vapor is a very different type of air constituent than oxygen and nitrogen. Oxygen and nitrogen are always gases at Earth temperatures, having boiling points of 90K and 77K respectively. Practically, they always act as ideal gases. But extraordinary water has a boiling point of 100°C= 373.15K and can exist in solid, liquid and gaseous phases on the Earth. It is essentially always in a process of dynamic exchange of molecules between these phases. In air at 20°C, if the vapor pressure has reached 17.54 mm Hg, then as many water molecules are entering the liquid phase as are escaping to the gas phase, so we say that the vapor is "saturated". It has nothing to do with the air "holding" the molecules, but common usage often suggests that. As the air approaches saturation, we say that we are approaching the "dewpoint". The water molecules are polar and will exhibit some net attractive force on each other and therefore begin to depart from ideal gas behavior. By collecting together and entering the liquid state they can form droplets in the atmosphere to make clouds, or near the surface to form fog, or on surfaces to form dew.
Another approach which might help clarify the point that air does not actually "hold" water is to note that the relative humidity really has nothing to do with the air molecules (i.e., N2 and O2). If a closed flask at 20°C had liquid water in it but no air at all, it would reach equilibrium at the saturated vapor pressure 17.54 mm Hg. At that point it would have a vapor density of 17.3 gm/m3 of pure water vapor in the gas phase above the water surface. But if you had just removed the air and sealed the container with liquid water in it, you might have a situation where there was only 8.65 gm/m3 resident in the gas phase at that particular moment. We would say that the relative humidity in the flask is 50% at that point because the resident water vapor density is half its saturation density. That is exactly the same thing we would say if the air were present - 8.65 gm/m3 of water vapor in the air at 20°C represents 50% relative humidity. Under these conditions, water molecules would be evaporating from the surface into the gas phase faster than they would be entering the water surface, so the vapor pressure of the water vapor above the surface would be rising toward the saturation vapor pressure.
|
Index
Kinetic theory concepts
Applications of kinetic theory
Vapor application concepts |